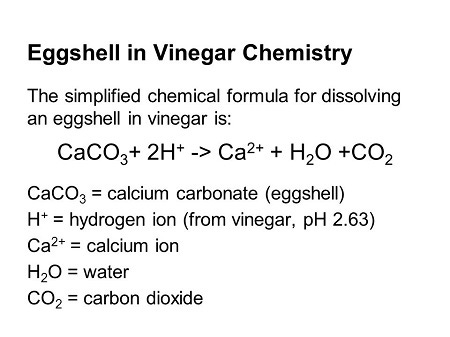From Minerals: Exploring Abiotic Korean Natural Farming
Exploring the chemical framework behind the poorly understood practice
Any time I hear someone talk about “natural farming”, my ears perk up and my suspicions tend to grow, and for good reason. “Natural farming” often falls into naturalist fallacies and a refusal to engage with replicable science but instead relies on ‘logical solutions’ that are poorly framed and often without any basis in basic science. Needless to say, when I first heard of Korean Natural Farming, I was suspicious and critical. However, the science behind much of KNF is actually quite solid.
Now, if we back up a bit, we talked about the microorganisms within the soil quite a bit here, here, here, and here, but now I want to take everything we’ve covered in the agricultural episodes and bring them back to the idea of building from the ground up. If we think about ecosystems, they operate similarly to a pyramid in that you’ve got biology in the soil, and that is the main function of a plant’s ability to grow to its fullest potential. The minerals in the dirt are, of course, important, and in many cases limit the capacity of what can grow and how large it can grow, and the biology operates as essentially the throttle to the whole system; they make everything accessible to the plants. So we’re going to talk a bit about Korean Natural Farming.
The term Korean Natural Farming stems from the work of primarily Cho Han Kyu, or Master Cho. The fundamental insight of KNF is to strengthen the biological functions of every aspect of plant growth to increase productivity and nutrition. Biology then reduces or eliminates the need for chemical interventions, whether to protect against predation and competition with other plants.1 Further, KNF works to utilize traditional fermentation methods to make soluble nutrients and to keep the biology that is able to make those nutrients soluble alive until utilized on a farm or garden site.
Now, Natural Farming has been trialed and supported by the South Korean government after successful trials of growing rice in one county, where every farmer followed the practice.2 These farms showed increased yields and saved money on inputs, and rivers and coastal waters experienced environmental benefits. Now, these farms weren’t simply trying new techniques based on Master Cho, but rather were continuing a long history of utilizing tools that have been in use in Korea for at least 500 years as outlined in “Concise Farming Talks”.3
There are two primary components of KNF, and today we’re going to focus on the mineral component of KNF. We had brought up the pyramid where there’s this base of basic nutrients within a soil, and it’s the biology that can convert it into plant-digestible nutrients, so the biology is incredibly important, but obviously, it’s necessary for the raw minerals to exist within the soil or the biology has nothing to work with. Fortunately, as long as there’s dirt, there will be some minerals available. What we can do with practices like KNF is to accelerate the natural restoration of these minerals within a specific site.
Before we jump in, I want to address my biggest concerns with Korean Natural Farming. There have been many folks who have taken Korean Natural Farming and, like any new field of study, over-exaggerated its ability. We try to not fit into that, and frame as much as we can within an evidence-based framework. So you may notice this episode has a lot of peer-reviewed research papers listed in the sources. Korean Natural Farming has a lot to offer us in the meantime, and our expectation shouldn’t be to cast aside anything that isn’t perfect but to find the positive things we can do that reduce destruction, accelerate our ability to heal our ecosystems, and in this case, do something that is incredibly cheap and is a great way to get sucked into science in a fun way. There are unique benefits of KNF over, say, JADAM, and there are better uses for one of the other depending on things like the length of your growing season, the resources for storage available, and so on. And we’re gonna cover all that.
When we talk about Korean Natural Farming, there are essentially two goals; to create more soluble nutrients in the soil for the biology and the plants and to increase the amount of biology in the soil to accelerate the biology’s ability to feed the plants more quickly. The first one is the one that I think is the easiest to understand and it’s the one we’re gonna focus on today. We talked about how there are a lot of nutrients often locked in the soil that the plants don’t have the ability to access because the nutrients are insoluble, and that’s why the relationships between fungi, bacteria, and nematodes are so important for them. What we’re doing is trying to create soluble nutrients for the plants by breaking down various matter. This can include plants and other things like eggshells, oyster shells, bones, seawater, and more, depending on what you’re trying to add to the soil. Shameless plug, if you haven’t checked out our YouTube channel, we’ve compiled a few how-tos for some of these practices, and is a great, accessible way to make your soil more resilient in the face of climate change.
The whole process is similar to what we had talked about with practices like intensive grazing and even fire management; we’re trying to accelerate nutrient return to the soil. While, say, bones and shells and rocks are made up of important minerals that our plants need, those minerals leech out naturally at an incredibly slow pace; in some cases thousands of years.4 We don’t have that kind of time, so we can use natural chemical reactions to accelerate this process.
So, let’s start with the non-plant sources of various nutrients. In my experience, calcium is not only the easiest to make bio-available for plants, but it’s also the one that makes the most sense to someone who is learning about this for the first time. Calcium is a common substance but the majority exists in the form of calcium carbonate, which cannot be directly absorbed by plants. When calcium is bonded to other minerals, it reduces its ability to be absorbed. This can happen in clay soils that are too low in pH (4.5 and below) as the calcium bonds to clay particles that roots cannot access them. Adequate calcium prevents overgrowth, firms fruit, prolongs durability, promotes the absorption of phosphoric acid, helps crops to accumulate and utilize nutrients, is the major component in forming cell membranes, enables smooth cell division and removes harmful substances by binding with organic acids.5
Water soluble calcium, written often simply as WSCA is produced by grilling and crushing cleaned egg shells and steeping them in vinegar until no bubbles are present. The bubbles indicate that the vinegar is reacting with organic matter to produce Co2. This process, using fire to break down the bonds within the materials and then treating them to a chemical reaction, is the process most common in utilizing any of the inorganic material in Korean Natural Farming. The general idea is to isolate the inorganic matter from the bonds with the organic matter, and to make it bioavailable for quick utilization on your site. The added benefit of this process is that it also creates a lot of accessible material; a couple dozen eggshells can create enough water-soluble calcium for a few acres for at least a year.
Generally, what you’ll want to do is cook down the eggshells or clam shells or oyster shells— whatever you’re using, until it’s browned and breaks fairly easily, almost like it’s charred. At this state it’s able to react with the low-pH vinegar to break those bonds and become water-soluble, and ultimately plant-soluble in the process. At a ratio of 10:1 vinegar to inorganic matter in volume, mix them in a jar and watch the bubbles take off as the chemical reaction kicks off. Total exposure to the vinegar is key, and occasionally moving the shells around over the course of a week or two will get you the full benefits of this process. Generally, these water-soluble minerals are used diluted at around 1000:1 ratios, about a teaspoon per gallon of water, and are used most often weekly as a foliar spray, that is, spraying the leaves, or as a soil drench, that is, watering the roots, or even both. In testing, this showed a 15% increase in sugar for grape production.6
Besides water-soluble calcium, another major nutrient that plants are often deficient in is calcium phosphate. Calcium-phosphate is soluble in vinegar, but much less so in water.7 Calcium-phosphate is available in bones, so it’s a great way to recycle materials that otherwise end up in the trash.8 It’s the same exact process as making water-soluble calcium. So what’s the importance of Calcium-phosphate? When calcium bonds with phosphorus to create phosphate of calcium, the phosphate brings the calcium through the plant and drops it where it belongs. It transports all nutrients throughout the plant with the exception of nitrogen.
For example, it promotes more photosynthesis and higher brix. The phosphates not only help produce the sugar but also bring it to the roots where it’s excreted to soil microbes. Then, the microbes make more nutrients that become available to the plant, so the plant can make more sugar. The challenge, however, is that even if we understand chemically what’s happening, there’s been little research done at the academic level to prove or refute these practices, specifically the measurements and guidelines, to back these practices— even if I have personally used them with success. But we do have some tools to apply in our own testing.
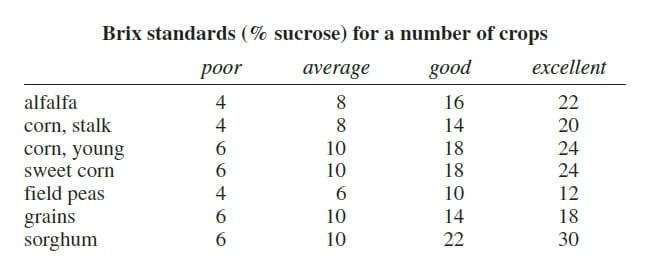
Degrees Brix (or Brix for short) is a term most gardeners are familiar with— it measures the level of sugar/sucrose in produce. A refractometer is used the measure the brix level; the higher the degrees, the sweeter the bite. Scales differ based on the produce being measured, but the general idea is that the higher brix, the less water-saturated a food is, and by proxy, the more full of, well, other stuff, usually meaning good stuff, that your food has. So more phosphorus, for example, can help process and store more sugars and other nutrients.
Now, brix isn’t just reflective of sugars, but all non-water solids within a specific plant, fruit, or whatever. However, carbohydrates, for example, can impact a brix number much more significantly than a large percentage change in a specific vitamin, for example, because of the simple fact that there are much fewer vitamins in something than sugar. So, theoretically, you could change growing conditions to double the amount of vitamins, but you might not see a significant change in the brix reading. This is true for most minerals. The biggest factor on Brix is water.9 More water means that a sample will be diluted, so while a lot of folks will point to increased brix readings as evidence of more nutrition or a healthier plant, that’s not necessarily the case. If you don’t water a tomato plant for a couple of days, suddenly the brix reading will have a less water-saturated figure. Is it healthy? No, it’s dehydrated. Even temperature changes can impact brix readings.10 My point here is that there are a number of pieces involved in what a brix reading might show you, and it’s worth learning this in more depth if you choose to utilize this tool.
Now brix readings are really helpful in knowing sugar content as a way to understand a plant’s ability to store energy when comparing two plants near each other with similar soil types that may be getting managed in different ways. Say, two tomato plants, one treated with KNF and one with conventional methods— if they’re the same size roughly, and the brix readings are different, that gives you some good insight into whether or not the plant is creating more dense foods, which may or may not actually be healthier, but at the very least are more delicious. Research has actually been going on showing that lower brix levels are often tied to low phosphate levels, while appropriate brix levels, according to the specific plant species, indicate relative resistance to bacterial, fungal, and insect attacks.11 Not only this but often low-brix flowers will get visited less often, if at all, by pollinators because it’s not worth the effort for the pollinator.12
So the last soluble amendment I want to talk about is water-soluble potassium. This shouldn’t be a surprise, since we’ve covered three of the 4 main nutrients that we think of when we think of plant amendments— NPK is nitrogen, phosphorus, and potassium—the 4th one usually referenced is calcium for the reasons stated above. Yeah, we haven’t covered Nitrogen— don’t worry; we’ll get there.
As for water-soluble potassium; the process is pretty similar, again, to calcium and calcium phosphate. We char thick green stalks— typically tobacco, sunflower, or cannabis stalks, but there’s probably more that could be used, break them into small pieces that can be stuck into water at a 10:1 ratio for a week. Strain and use at a 1:1000 ratio, or one tsp per gallon. Potassium is associated with the movement of water, nutrients, and carbohydrates in plant tissue, and because of this it resides in the remainder of the straw or stalks of annual crops, keeping the plant upright well after it has died.13 It’s involved with enzyme activation within the plant, which affects protein, starch, and adenosine triphosphate (ATP) production. The production of ATP can regulate the rate of photosynthesis.14 Potassium also helps regulate the opening and closing of the stomata, which regulates the exchange of water vapor, oxygen, and carbon dioxide.
Now, we’re not gonna cover nitrogen support in KNF in this episode, because it’s part of a bit of a more complicated process related to the abiotic amendments. Still, we’ve found solutions to address many of the challenges of amending the soil in terms of the main nutrition plants need to be successful.
While these different byproducts of other living things reflect the shorter-term cycling of minerals into the soil through living things, these aren’t the only ways to get minerals returned to the soil. We can use things such as rocks, which contain trace minerals, which can be used in particular for the long-term mineralization of soil. This is probably one of the more intimidating processes because we often think that, well, most of the rock in my soil comes from massive boulders, which means it’s pretty homogenous, right? Well, maybe, but also probably not.
You can use local geological survey maps to help identify minerals below the ground surface. These types of maps will specifically identify the rock types in your area. Now, if you’ve done a soil sample, you should be familiar with what’s already on your site, and comparing these results with the rocks that show up in your geographical survey can offer an opportunity to see what’s available locally that can help improve the mineralization on your site.

Depending on where you live, it’s worth checking out if there are any quarries nearby. These sites are particularly useful because they build up rock dust from crushing, and they’re often happy for someone to take some of it off their hands for free. This doesn’t mean take any and all rock materials because you may not need what’s available; this is just the first step in trying to find out what’s available nearby as a first choice to adding those choice minerals. You can always buy rock dust, and many of them are commonly available online.
Now if you’re struggling to find a geological survey map from the United States Geological Survey, the USGS released a paper called “Element Concentrations in Soils and Other Surficial Materials of the Conterminous United States”, which is linked here, which provides a bunch of information about mineral content of the soil at a state level.
So how do we add these to the soil? If we do have access to crushed stone dust to add, we can add the dust particles right into our soil surfaces. If you have a large pile of compost, adding to the compost is great as well, because in order for the minerals to become available to plants, it’s necessary for the soil biology to digest the rock dusts, which is unsurprisingly a slow process.15 Much like adding lime to your front yard takes 6 months to impact soil pH, these rock dusts take quite a while to make a difference. That said, researchers are actively looking into using silica and other minerals as an alternative to conventional fertilizers with promising results, even in shorter timeframes.16
In the same vein as rock minerals, silts and clays also offer unique benefits, depending on the site in particular that we’re working on— and we discussed silts in particular during Franklin Hiram King’s work writing about farmers in China in the 19th century. Of course, when it comes to taking silts in particular, it’s important to be aware and thoughtful about how much you’re removing from the site since silts are particularly important in places like river basins and in ponds. It’s also important, if you can, to consider doing a soil analysis of the silts to see what minerals are present, and of course if you live somewhere like the Northeastern USA, to make sure heavy metals aren’t collected in the silts.
Silts are often loaded in things like nitrogen and anaerobic bacteria that will quickly break down when added as an amendment to topsoil, releasing nutrient-rich, easily digestible nutrients to your soil.17 Harvesting silts are one of the most ancient farming amendment practices in human history and is a core piece of how annual agriculture came to be, so don’t miss the opportunity to utilize this ancient practice.
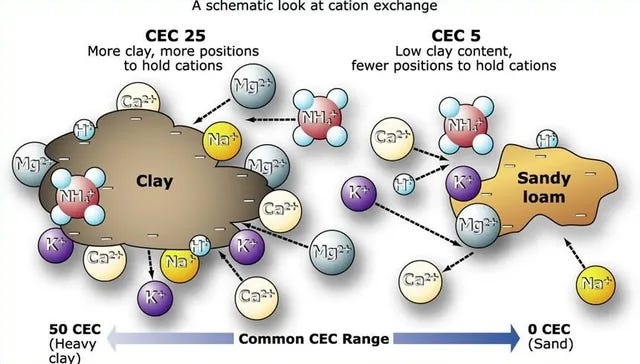
Further, on the opposite end of the spectrum in many ways, is the harvesting of clays for your soil, especially if you have naturally sandy soil like I do. We’ve talked a bit about how sandy soils drain well, but the downside is that minerals and nutrients are easily washed away because of the large particle size of the sand grains which allows for easy loss of material. Well, that process is actually a little more complicated. There’s a measurement system called the cation exchange capacity, and it’s a measure of a soil’s ability to hold positively charged ions.
Clay soils and soils with high amounts of organic matter have negatively charged sites on their surfaces which absorb and hold positively charged ions— called cations— by electrostatic force. Remember back in high school the positive and negative ions? Yeah, that, this electrical charge is super critical to the supply of nutrients to plants because many nutrients exist as cations (potassium and calcium being 2 we’ve already talked about). In very general terms, soils with large amounts of negative charge have more nutrients because they retain more of these positively charged ions.
Now, this all ties into the larger conversation on balancing soil pH, understanding why sandy soils struggle so much, and so on. Clay soil actually has a slight magnetism, and this is part of it. Increasing the soil’s cation exchange capacity by increasing the organic matter or by adding clay to your site, whichever is more accessible, helps to build that capacity to retain nutrients by reducing the ability of water to flow through the site using smaller grained clays and by increasing that electrostatic force. However, be wary about just dumping clay into sandy soils, as it can make the soil much harder to work with.18 Think of it this way, if sand particles are apples in a bucket, then clay would be like adding sand to that same bucket. Would it be easier to move your hand around in a bucket full of apples or a bucket full of apples and sand? If this is something you want to try, keep the clay to a minimum. You’re far better off building organic matter instead and considering the longer-term horizon about what the site is designed to grow and working in alignment with this.
If you live near the ocean, there’s an incredible amount of resources available for soil amendments, starting with the ocean water itself. Sea water can be diluted and applied to our soils, pastures, and even the plants themselves, as the water is rich in trace minerals— at least 47, according to researchers at Stanford.19 Typically, seawater is diluted at a 50 to 1 ratio, so for every cup of seawater, you’ll mix with 50 cups of rainwater. This can be sprayed every few weeks if possible; the biggest challenge is keeping salt build-up from occurring.
Not only can we use seawater, we can harvest salt from the ocean and use the salts to create salt licks for our livestock! This isn’t really a KNF thing, but since we’re talking about using the ocean’s resources, it’s worth bringing up.
And of course, there are the various crustaceans whose shells are full of minerals. When you’re done eating them, you can crush their shells and break them down into water-soluble calcium just like eggshells. We can use seaweeds for Fermented Plant Juice (which we’ll be covering in the next piece), and we can even use fish byproducts we catch into Fish Amino Acids.20
Fish amino acids are processed using brown sugar at a 1:1 ratio, and have a long history of use in adding nutrients to soil, and is backed extensively by science.21,22 In the next KNF piece, we’ll explain why brown sugar, as we’ll be talking about utilizing it more, but for the purposes of this piece, we’ll continue on. Chopping the fish up into chunks, mix it all together so the fish is completely covered in the brown sugar. Add it to a bowl or box so that it’s 2/3rds full. Cover the top of the mixture with a layer of organic brown sugar, which will form a cap as it dries out and reduces concerns about outside contaminants getting in. If you have homemade compost or IMO-4 available, add a handful to the top and this will help accelerate and eliminate some of the smells of the fish breaking down. Cover with a cloth and ferment for about 6 months in a dry, well-ventilated place at about room temperature. After 6 months, a liquid will be collected at the bottom of the bowl and that’s your fish amino acid. The rest of the fish can go in the compost, or you can do a vinegar extraction to help isolate some of the other nutrients in the fish as another amendment.
Much like the other resources, FAA is applied at a 1:1000 ratio, or a little less than a tsp per gallon, and; if we think about what fish is primarily composed of— protein— it shouldn’t be much of a surprise that FAA is treated like a nitrogen-dense fertilizer.23 The Republic of the Philippines Agricultural Training Institute lists that the liquid drained from the process is 90% nitrogen and 2.5% phosphorus.24 I haven’t seen a secondary source or even where the research came from, it’s from an old pamphlet, but it would make sense in terms of its strength and dilution.
There are benefits to collecting amino acids from other things we can harvest; for example, if you butcher a chicken and you’re not a huge fan of eating their livers, liver amino acids are another, smaller-scale alternative to fish amino acids. Of course, this speaks to the opportunity to experiment in the KNF field, as there is not one paper I can find on the nutritional breakdown of liver amino acids, but given that liver is probably as close to a superfood as exists on the earth, sorry vegetarians, I have to imagine the spectrum of nutrients extracted are incredibly beneficial. Liver is high in protein, so it’s going to provide a ton of nitrogen, while ratio-wise it will likely be higher in potassium in comparison to fish. Needless to say, there’s an opportunity in resources available for you if you start thinking outside the box, and Korean Natural Farming provides a basic framework to start experimenting from. The key is to understand the thought processes, which helps you start thinking about what’s available to you in new ways.
In this piece, we have focused primarily on abiotic ingredients, which is probably the less commonly discussed side of Korean Natural Farming. Part of this decision to start here was because I think it’s much easier to understand, and then we can essentially take that framework we’ve discussed here and apply it to fermented living material and plant extracts. We’ll dig in deeper in the next piece to what exactly is happening with the brown sugar application, harvesting indigenous micro-organisms, and more.
The last thing I want to mention is that Korean Natural Farming is still not heavily researched, at least in English-speaking parts of the world. I’ve tried to cite this article with as much evidence as possible, and resources are limited for proof. Some of it is simple chemistry, which isn’t an active area of research for obvious reasons, and some of it simply doesn’t have the funding behind it to research. This can and should be of interest to folks studying agriculture in school since the research needs to be done, whether it proves or disproves the ability of KNF to deliver on what it promises, even if the anecdotal evidence seems to point to the former. Not only does research need to be done on whether or not it works, but also the application rate, the shelf life of these products, and more need to be better understood, and that’s where you personally can contribute to a better future around agriculture.
If you’ve enjoyed this piece, which is equal to a 19-page chapter, of (so far) an 839-page book with 504 sources, you can support our work in a number of ways. The first is by sharing this article with folks you think would find it interesting. Second, you can listen to the audio version of this episode, #69, of the Poor Proles Almanac wherever you get your podcasts. Suppose you’d like to financially support the project, and get exclusive access to our limited paywalled content. In that case, you can become a paid subscriber on Substack or Patreon, which will both give you access to the paywalled content and in the case of Patreon, early access to the audio episodes as well.
Ramasami, P., Gupta-Bhowon, M., Jhaumeer-Laulloo, S., & Henri., L. K. W. (2018). Emerging trends in Chemical Sciences. Springer International Publishing.
https://www.ipcc.ch/site/assets/uploads/2019/08/4.-SPM_Approved_Microsite_FINAL.pdf
https://www.longdom.org/open-access/a-story-of-globally-important-agricultural-wisdom-in-the-15th-centurychosn-korea-2332-0915-1000202.pdf
https://lawr.ucdavis.edu/classes/ssc219/biogeo/weath.htm
https://lubbock.tamu.edu/files/2011/10/calciumplantgrowth.pdf
Wang, R., Qi, Y., Wu, J., Shukla, M. K., & Sun, Q. (2019). Influence of the application of irrigated water-soluble calcium fertilizer on wine grape properties. PLOS ONE, 14(9). https://doi.org/10.1371/journal.pone.0222104
https://www.deseret.com/1990/10/2/18883976/test-solubility-of-calcium-by-using-vinegar
https://lawrencehallofscience.org/wp-content/uploads/2021/10/dino_ex1.pdf
https://www.fruitsmart.com/wp-content/uploads/Brix-Table-USDA-Conversion-Chart.pdf
https://www.mt.com/us/en/home/perm-lp/product-organizations/ana/brix-meters.html
https://cesablog.co.za/2021/07/14/brix-measurements-and-its-role-in-plant-nutrient-management/
Aronne, G., & Malara, P. (2019). Fiber‐optic refractometer for in vivo sugar concentration measurements of low‐nectar‐producing flowers. New Phytologist, 224(2), 987–993. https://doi.org/10.1111/nph.16084
https://grow.ifa.coop/agronomy/potassium-regulating-power-in-plant-growth-health
https://extension.umn.edu/phosphorus-and-potassium/potassium-crop-production#:~:text=The%20production%20of%20ATP%20can,plant%20growth%20and%20reduces%20yield.
https://www.ctahr.hawaii.edu/mauisoil/a_factor_form.aspx
Swoboda, P., Döring, T. F., & Hamer, M. (2022). Remineralizing soils? the agricultural usage of silicate rock powders: A Review. Science of The Total Environment, 807, 150976. https://doi.org/10.1016/j.scitotenv.2021.150976
Palmer, N. (2020). The Regenerative Grower’s Guide to Garden Amendments: Using locally sourced materials to make mineral and biological extracts and ferments. Chelsea Green Publishing.
https://extension.illinois.edu/blogs/good-growing/2018-01-31-does-sand-improve-clay-soil-drainage
Sharkh, B. A., Al-Amoudi, A. A., Farooque, M., Fellows, C. M., Ihm, S., Lee, S., Li, S., & Voutchkov, N. (2022). Seawater desalination concentrate—a new frontier for sustainable mining of valuable minerals. Npj Clean Water, 5(1). https://doi.org/10.1038/s41545-022-00153-6
http://organic.da.gov.ph/images/IECs/FAA2.pdf
Kalajahi, Bahman. “Response of sunflower (Helianthus annuus L.) to plant nutrition systems in the soil under foliar spraying by amino fish AMI-16, fulvic acid and microfertilizer”. https://www.researchgate.net/publication/360439077_Response_of_sunflower_Helianthus_annuus_L_to_plant_nutrition_systems_in_the_soil_under_foliar_spraying_by_amino_fish_AMI-16_fulvic_acid_and_microfertilizer
Bhuimbar, M. V., & Dandge, P. B. (2022). Production of organic liquid biofertilizer from fish waste and study of its plant growth promoting effect. Proceedings of the National Academy of Sciences, India Section B: Biological Sciences, 93(1), 235–243. https://doi.org/10.1007/s40011-022-01413-8
Krishnamoorthy, R., Alshatwi, A. A., Subbarayan, S., Vadivel, B., Periyasamy, V. S., Al-Shuniaber, M. A., & Athinarayanan, J. (2018). Impact of farm-made liquid organic nutrients jevamirtham and fish amino acid on growth and nutritional status in different season of Abelmoschus esculentus—a self-sustainable field trial. Organic Agriculture. doi:10.1007/s13165-018-0205-2
https://ati2.da.gov.ph/ati-car/content/sites/default/files/2023-01/MOTHER%20NATURES-ORGANIC%20CONCOCTIONS.pdf