Examining the Relationship Between Microbes and Nitrogen in Soil
The Nitrogen Cycle and the role of bacteria and fungi
So we all know fungi and bacteria are crucial for plant life, but what do we actually know about them? Plants grow in soil, where they obtain nutrients through exudates, particularly those produced by bacteria and fungi. These fungi then produce substances that are soluble in water and accessible to plants. Protozoa and nematodes attempt to feed on bacteria and fungi, which also makes more nutrients available in the soil for plants. Arthropods then eat the protozoa and nematodes.
Everything eats everything, and the size of the animals doing the eating keeps getting bigger, but it all comes back to the plants, which can photosynthesize light, right? And there’s the added benefit of organisms like bacteria, fungi, worms, and ants, which, when they work through the soil, either produce a slime to help stabilize the living thing or burrow, creating pathways for air and water to enter and leave the soil. All of this together creates both spongy, glued soil that’s also airy. And when any of these pieces die or are eaten, the nutrients that they’ve consumed return into the soil. This diversity also keeps bacteria and fungi, which can be considered detrimental to plants, in check.
However, this ratio varies depending on the needs of the plants; remember, the plants drive this process because they’re the ones capturing the energy from the sun and incorporating it into the system. This is why old-growth forests have fungally dominated soils, and early-stage succession sites are bacterially dominated; this exists on a continuum. I think this summarizes the first two pieces we did on soil.
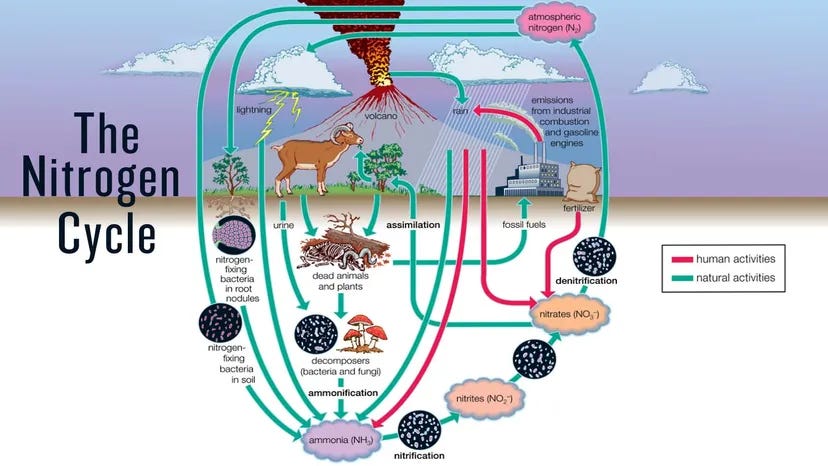
Let’s delve a little deeper into the impacts of microbes, specifically on nitrogen. When these various organisms are consumed, the predator retains some of the nitrogen present in the prey. At the same time, some of it is released as waste in the form of plant-available ammonium. It serves as a food source for special bacteria that can convert it into nitrates, and these bacteria thrive in high-pH, bacterially dominated soils. These alkaline soils are characterized by the presence of bacterial bioslime, which enables nitrogen-fixing bacteria to thrive.
First, commercial fertilizers primarily produce nitrate, which is suitable for high-pH soils. Now, to fully understand and appreciate the role microbes play in this space, we need to recall the JADAM content.
Previously, we discussed cation exchange capacity, which refers to the ability of soil particles to bond with minerals and water. Generally speaking, the lower the pH, the more difficult it is for the soil to hold these minerals through magnetic bonds. Beyond pH, sandy soils also tend to have less charge. Therefore, they have a harder time holding minerals. Keep in mind that lower pH soils are more common in forests, but those same forests also have high amounts of organic matter, which share similar properties. The reason this cation exchange capacity is essential, though, for what we’re talking about, is that while being able to bond with, say, water is excellent for plants, it’s more important for bacteria. These bonds are often between the soil particles and hydroscopic water, which is only a few molecules thick, and this super-thin film creates such a strong bond that the plants can’t access it.
This collection of super-thin water that spreads across the entire soil is crucial for the survival of microorganisms. The bonds in this water are so strong that even drought often doesn’t break them, unless the soil is completely unprotected on hot days. Even dry soil, where plants are dying, will still contain this hygroscopic water. And of course, with this water, there needs to be air, right? Otherwise, anaerobic bacteria will thrive, and their byproducts, like alcohol, will eventually kill plant roots. Not ideal.
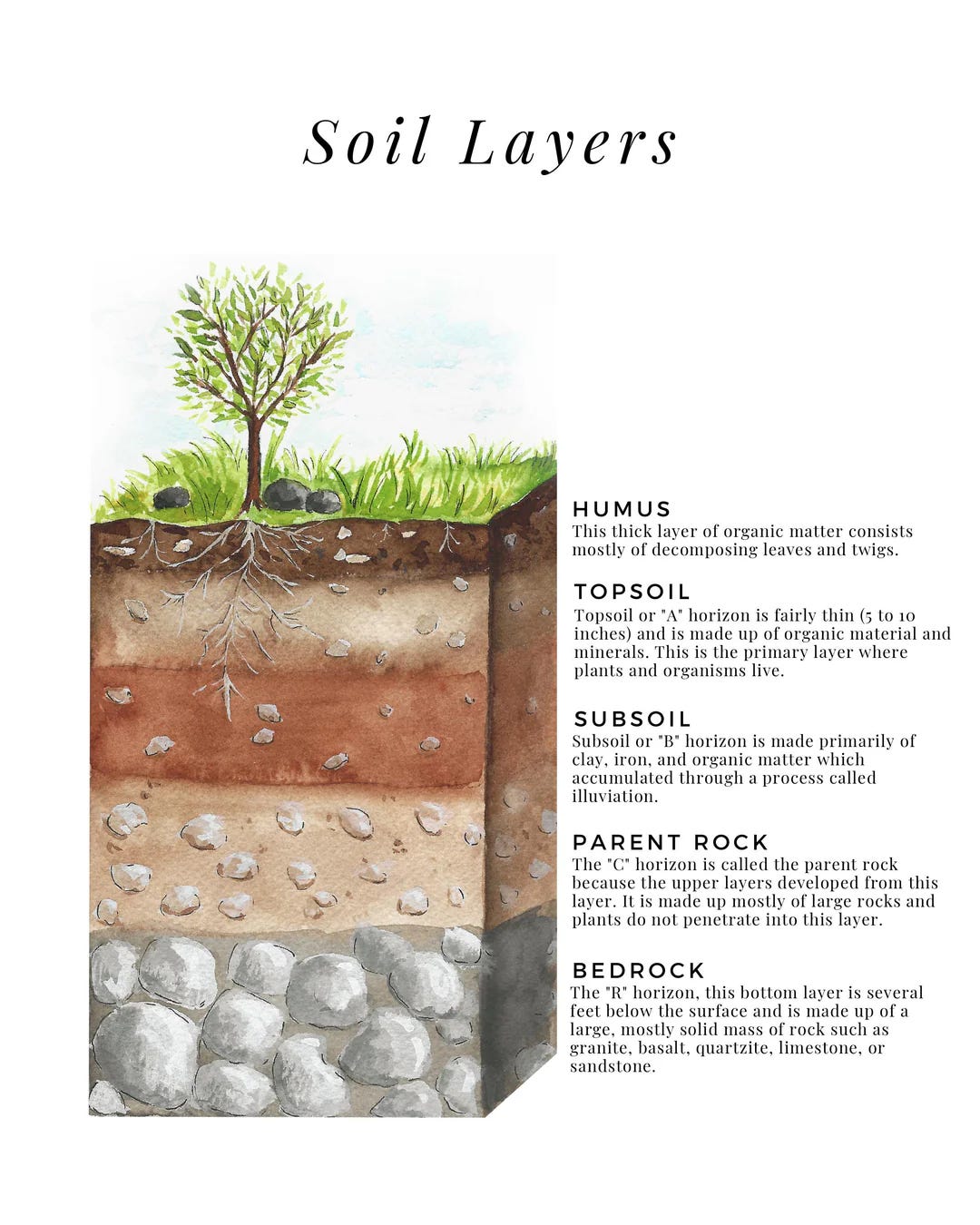
Before we can explore this concept further, I would like to discuss soil horizons briefly, and then we can delve deeper into the subject. *break* get in deep– soil horizons.. Get it?
Everyone is familiar with the concept of soil horizons, but instead of rehashing that subject, I would like to delve a bit more into the top horizon. If you’re familiar with undisturbed soils in a forest, the layers of the topsoil are pretty obvious. You’ve got your Oi horizon, the surface layer, where you’ve got the duff, the stuff that’s still clearly plant or animal material.
Below this is your Oe layer, which is further broken down to the point where you know it’s plant material but can't identify it, and Oa is where it’s so decomposed that you can’t tell if it’s animal waste or plant remains. The reason you need to know these layers is so you can determine if your soils will produce more of those decomposition by-products, such as nitrogen.
We can, by observation, understand how our soil will evolve in terms of nutritional content through this “work-in-progress” layer. We can also determine the health of our soil by the amount of polysaccharides, the sticky substance that bacteria, fungi, and worms use to bind minerals and humic particles together, that exists.
To circle back, when we talk about pH, what we’re talking about is the concentration of hydrogen ions in the solution being measured. The reason I’m bringing this up is because I think people understand pH as this abstract thing that is kinda related to fungal/bacterial ratios, which it is, but there’s a chemical basis for it. And what we see when plants exchange hydrogen cations for nutrient cations is that the concentration of hydrogen cations in the soil increases, meaning the pH decreases, which is why forests are almost always low pH. And they are often dominated by fungi because fungi can better tolerate low-pH soils.
This does get somewhat balanced out because roots also take up negatively charged anions, which raise the pH back up a bit, which is probably why soils don’t become overwhelmingly low in pH even as they enter an old-growth stage.1 That’s the chemical composition. Now we know that fungi and bacteria make up the bulk of the foundation of the soil food web. Let’s start with bacteria, as they are more prevalent in early succession, which plays a significant role in soil development. Bacteria are the second most common primary decomposers of organic matter, breaking it down into minor, electrically charged pieces that store energy.
What’s important to understand is that the bacteria are capable of breaking the bonds along the chains of complex molecules. These are broken down into simple sugars, fatty acids, and amino acids —the three building blocks of bacteria. However, bacteria specialize in different ways. Actinomycetes, for example, use enzymes to produce healthy soil with its characteristic ‘earthy’ smell, while cellulomonas utilize cellulose-degrading enzymes to break down cellulose.
Meanwhile, woodier materials are composed of much more complex chains of interlinked alcohols, which are resistant to the enzymes produced by most bacteria and are only susceptible to those made by fungi. The point is that all of this is cyclical. Whether it's carbon dioxide, a byproduct of bacterial metabolism, which is then sequestered in plant and animal biomass only to return to the soil and be cycled back into the atmosphere through decay, or sulfur or nitrogen, all of these are cyclical processes directed by soil biology, not chemical reactions.
Now, let’s focus on nitrogen, as I wanted to discuss this the most, and how the microbial community affects the presence of nitrogen in soils. So we’ve talked about ammonium, right? This is where the process starts, the decomposition of proteins into ammonium. This is a waste product of bacteria and fungi getting eaten by nematodes & protozoa. Afterwards, special nitrate bacteria convert this ammonium into nitrites. A second type of special nitrate bacteria then converts nitrites into nitrates. These bacteria don’t like acidic environments, so they’re far more often found in soils with a pH above 7, much like the bacterial slime we mentioned earlier. And like our plants, bacteria strive to maintain an environment that meets their needs. Therefore, the more bacteria, the more slime, and the soil will remain above a pH of 7. If the soil has a pH of 5, very little of the ammonium is converted, locking up the potential nitrogen for the plants.
Now, unlike bacteria, fungi can grow in size like a plant, while bacteria can only grow in number, like the bacteria in the soil. Because of this, fungi don’t need a film or water like bacteria to travel through the soil. This allows them to find new food sources and transport nutrients; depending on the size of the fungus, this can occur across the entire fungal mass, which is often several yards in diameter. Additionally, like bacteria, they can enter a dormant state and await optimal conditions for growth.
Some fungi prefer the sugars that bacteria enjoy, although most are more efficient at breaking down more complex foods. While breaking down complex foods like cellulose, the fungi grow at a rate of 40 micrometers per minute, or approximately the entire growth cycle of most soil bacteria throughout their entire life.
We mentioned bacteria were the number 2 decomposer worldwide; well, fungi are number 1. Unlike bacteria, they can break down the tougher lignin and cellulose, as well as the shells of insects and even bones. Its ability to move nutrients while also reaching across the horizons of the soil make it uniquely crucial in building soil, and what’s particularly important, and why fungal additions to indoor grows is significant, is that it can break the bonds of phosphorus locked in soils, which even when added as a soil amendment often becomes immediately locked up. They’ll trade it with plants for exudates as the plant requests it.
So when fungi eat or die, a significant portion of what’s released in either process is nitrogen. We’ve talked about ammonium versus nitrate, right? When fungi release nitrogen, it’s in ammonium form. If those nitrifying bacteria are present, the process to convert them to nitrates is done, but that’s only in high-pH soils.
The enzymes released by fungi are acidic, which lowers the pH. So as the fungi grow, because they can grow significantly faster than bacteria, the populations of nitrogen-fixing bacteria shrink, making more ammonium available instead of the high-pH produced nitrates. In short, fungi and bacteria release different types of nitrogen and alter soil pH through their byproducts and volume, while plants also modify this process to obtain the specific kinds of nutrients and nitrogen they require.
Alright, so a few quick points. In soils without fungi or the bacteria that convert ammonium to nitrates, these two processes are absent, and the nitrogen will quickly be oxidized and lost. This is why building the soil is so essential; simply adding fertilizers is not a long-term solution. And way back two years ago, we summarized this, but generally speaking, early succession plants do better with the nitrates produced by bacteria, and trees do better with ammonium, which is more prevalent in lower-pH soils, where the fungal ratios are higher. The young plants die, providing more food for the fungi, which further increase that ratio. However, remember that there are still some bacteria, mind you, and even plants that prefer one type of nitrogen over another and can still utilize the less preferred type, just not as efficiently. Remember that the bacterial mass doesn’t significantly change; it’s just the fungal mass that’s increasing.
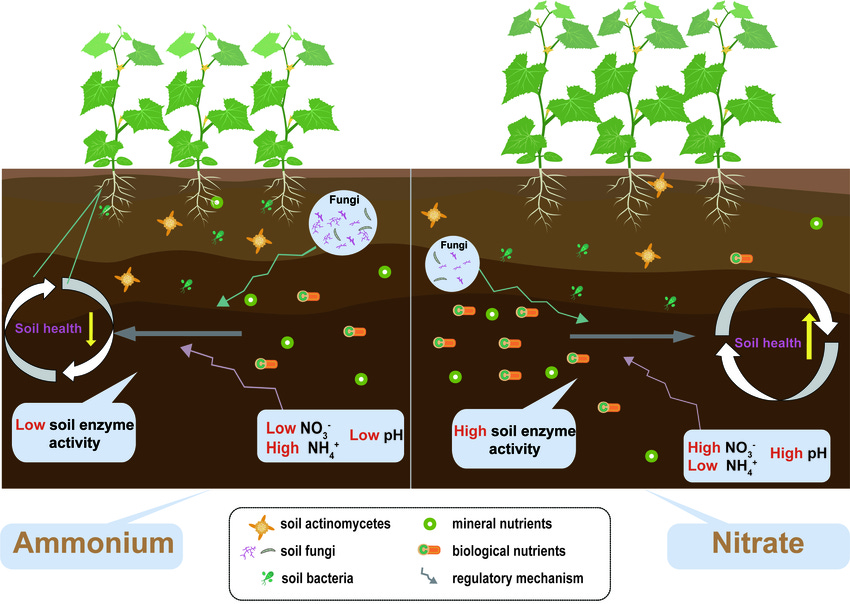
So, how do we utilize this knowledge in our gardens? Obviously, this is a bit more complicated than I can describe here. Still, basically, the higher the proportion of greens, such as grass, compared to leaves or wood chips, the higher the bacterial ratios, and conversely, this will impact the pH of the compost, right? We can build bacterial mulches using the same materials we’d use to make bacterial compost – lawn clippings and other green materials will create a bacteria-oriented mulch, which will be beneficial for your annual garden’s plants.
You can even shred some of those brown mulches, such as leaves, extremely finely to make the nutrients more accessible to bacteria, but it will still have a fair amount of fungal activity due to the materials. Jeff Lowenfels argues that the same mulch can be used differently; burying mulch can increase bacterial activity while surface mulching increases fungal activity. The reason for this, he argues, is that bacteria can’t easily travel like fungi can.
The idea of using bacterially dominated mulches, in particular, also works well in reducing weeds. The mulch takes time to break down and ties up nutrients on the surface in the short term, while also decreasing sunlight penetration into the soil, reducing weeding, and making the work easier overall. Sometimes you’ll hear the term ‘green mulch,’ and this is precisely what they’re talking about.
By no means is this an extensive analysis of what’s happening in the soil beneath our feet. However, it is sufficient to provide us with the tools necessary to make informed decisions regarding how we choose to manage the land and what the consequences of those decisions may be.
If you’ve enjoyed this piece, which is equal to a 9-page chapter of (so far) a 1433-page book with 1043 sources, you can support our work in several ways. The first is by sharing this article with folks you think would find it interesting. Second, you can listen to the audio version of this article in episode #131 of the Poor Proles Almanac wherever you get your podcasts. If you’d like to financially support the project and get exclusive access to our limited paywalled content, you can become a paid subscriber on Substack or Patreon, which will both give you access to the paywalled content and, in the case of Patreon, early access to the audio episodes as well.
Lowenfels, Jeff, and Wayne Lewis. Teaming with Microbes : The Organic Gardener’s Guide to the Soil Food Web. Portland, Or., Timber Press, 2016.